Understanding the Link Between Non-Coding RNA, Neuroinflammation, and Major Depressive Disorder
Major Depressive Disorder (MDD) is more than just a psychological condition—it's a complex interplay of genetic, epigenetic, and environmental factors. Emerging research highlights a critical role for non-coding RNAs (ncRNAs) and neuroinflammation in the pathophysiology of depression.
A recent study published in [International Journal of Biological Macromolecules (2024)] sheds light on how circular RNAs (circRNAs), long non-coding RNAs (lncRNAs), and microRNAs (miRNAs) contribute to neuroinflammatory processes that underpin depressive behaviors.
We gonna explore :
- The role of non-coding RNA in neuroinflammation.
- How circRNAs like circ_0000831 modulate glial activation.
- Implications for diagnosis and treatment of MDD.
- Tools like RT-PCR detection kits for studying these mechanisms.

What Are Non-Coding RNAs ?
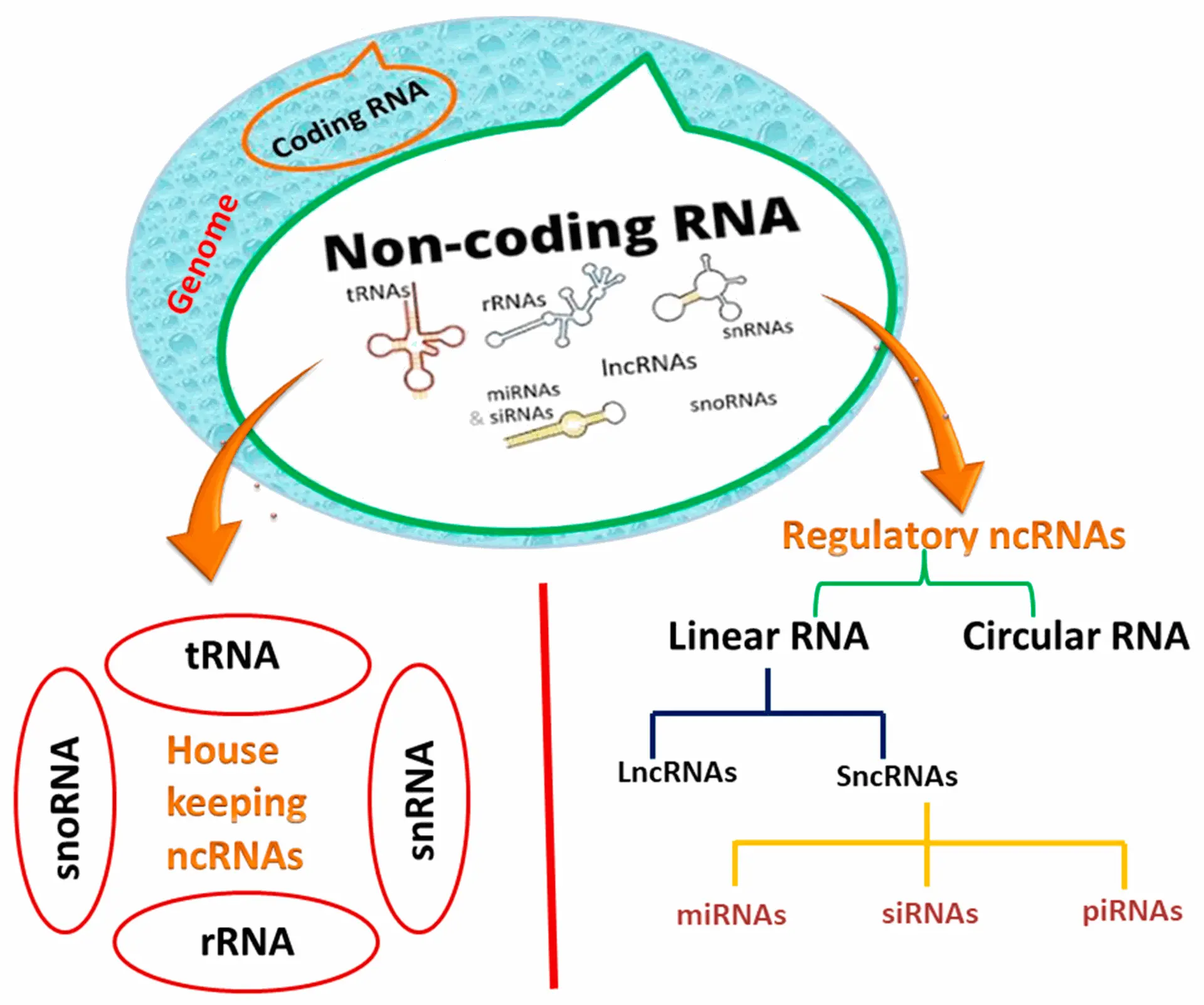
Non-coding RNAs are RNA molecules that do not code for proteins but play essential regulatory roles in gene expression. They include:
- microRNAs (miRNAs)
- long non-coding RNAs (lncRNAs)
- circular RNAs (circRNAs)
These ncRNAs have been increasingly implicated in psychiatric disorders, particularly through their influence on glial cell function , synaptic plasticity , and neuroinflammatory pathways .
circ_0000831 Suppresses Neuroinflammation via miR-16-5p
One of the most compelling findings from the 2024 International Journal of Biological Macromolecules paper is the discovery that circ_0000831 plays an anti-inflammatory role in cerebral ischemia-induced vertigo and may also be relevant in depression.
The study found that overexpression of circ_0000831 significantly reduced neuroinflammation by targeting miR-16-5p , leading to downstream suppression of inflammatory cytokines and improved behavioral outcomes.
This mechanism suggests that circRNAs could serve as potential biomarkers or therapeutic targets for inflammation-related depression.

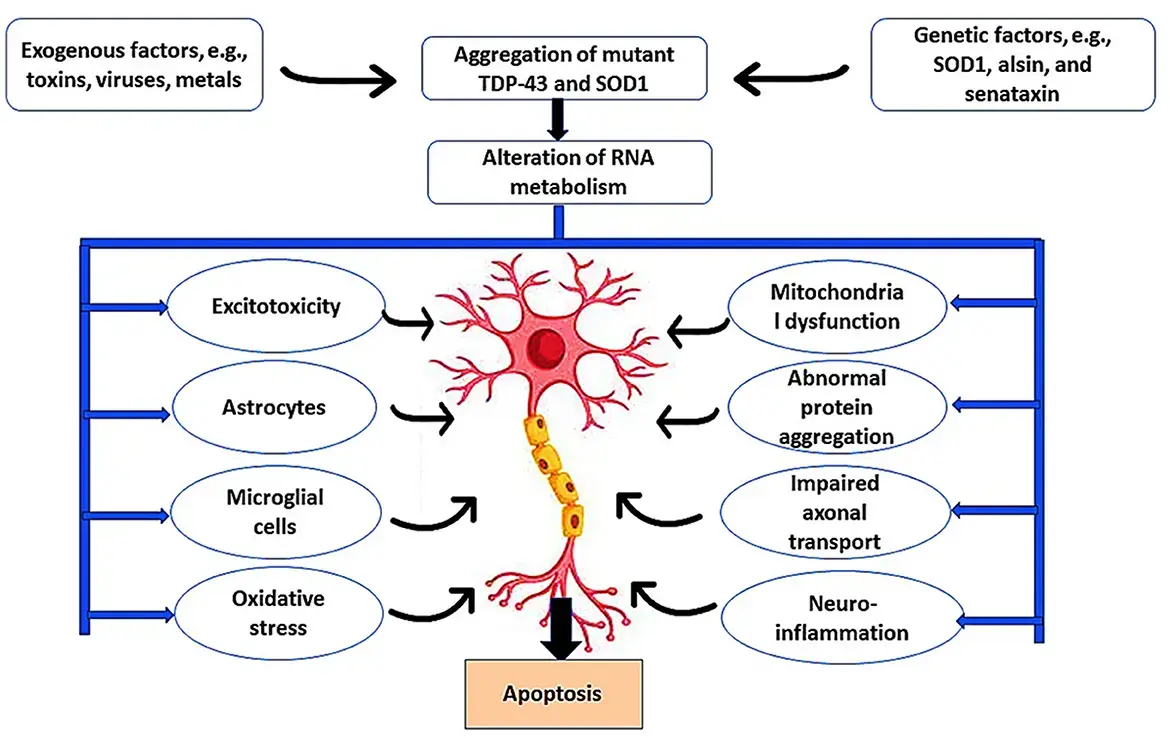
Neuroinflammation in MDD – A Key Player in Pathogenesis
Neuroinflammation is now recognized as a core component of major depressive disorder , especially in treatment-resistant cases. Inflammatory mediators such as:
- Interleukin-6 (IL-6)
- Tumor Necrosis Factor-alpha (TNF-α)
- Interferon-gamma (IFN-γ)
...are frequently elevated in patients with MDD.
Glia—particularly astrocytes and microglia —are key cellular players in this process. Activation of these cells contributes to synaptic dysfunction, neuronal loss, and impaired neuroplasticity.
Studies referenced in the paper, including Wu et al., 2019 and Tang et al., 2021 , demonstrate how interventions targeting astrocytic activation can alleviate depressive-like symptoms in animal models.
circRNAs as Potential Biomarkers
Because circRNAs are stable in bodily fluids , resistant to degradation by exonucleases, and exhibit tissue-specific expression, they are ideal candidates for non-invasive biomarkers of depression.
Tools such as the MDD CircRNA RT-PCR Detection Kit allow researchers to detect and quantify specific circRNAs like:
- circDYM
- circHIPK2
- circSTAG1
- circ_0000831
These tools offer a pathway toward precision psychiatry , where molecular profiling guides individualized treatment strategies.
Targeting Neuroinflammation for Novel Therapies
Anti-inflammatory agents, psychobiotics, and RNA-based therapies are emerging as novel approaches to treat MDD. For example :
- Malva sylvestris extract was shown to reduce astrogliosis and inflammation in LPS-induced depression models Wu et al., 2019 .
- HECTD1 inhibition has been linked to reduced astrocyte activation via the sigma-1R-JNK/p38-FOXJ2 axis Tang et al., 2021 .
These findings open up exciting possibilities for developing targeted treatments that address the biological roots of depression.