PCR Clean-Up & Gel Extraction Kit
– Fast & High-Recovery DNA Purification
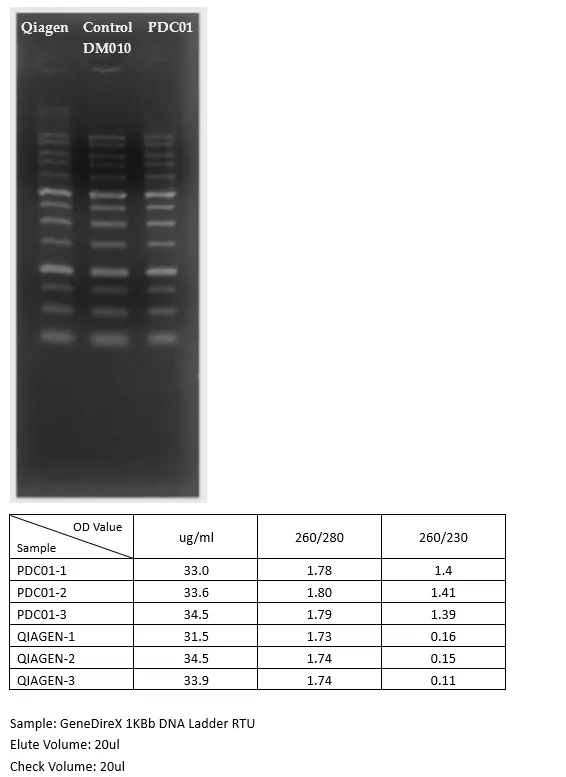
The PCR Clean-Up & Gel Extraction Kit offers a fast, reliable, and cost-effective solution for purifying DNA fragments from PCR reactions, agarose gels, and enzymatic digests. Designed for rapid workflows, this spin-column-based kit utilizes a glass fiber matrix to selectively bind DNA fragments ranging from 100 bp to 10 Kb, ensuring high purity without the need for phenol, chloroform, or ethanol precipitation.

Key Features :
- Dual Functionality – One kit for both PCR clean-up and gel extraction
- High DNA Recovery – Yields up to 95% of DNA fragments
- Fast Workflow – Complete purification in just 15–20 minutes
- Flexible Elution – Elute with Tris buffer or nuclease-free water
- Phenol-Free Protocol – Safe and clean, no organic extractions required
Product Specifications :
| Attribute | Description |
|---|---|
| Input Volume | Up to 100 µL PCR product or 300 mg gel |
| Fragment Range | 100 bp – 10,000 bp |
| Elution Volume | Typically 30–50 µL |
| Binding Matrix | Glass fiber column |
| Recovery Efficiency | Up to 95% |
| Time to Complete | 15–20 minutes |

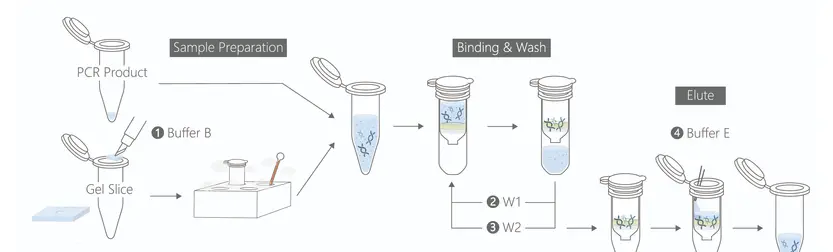
Protocol : PCR Clean-Up & Gel Extraction Kit
🔹 Step 1 – Sample Preparation
🧬 PCR Clean-Up
- Add 500 μL of Buffer B to 100 μL of PCR product.
- Mix thoroughly by vortexing.
🧬 Gel Extraction
- Excise the desired DNA fragment from agarose gel.
- Transfer up to 300 mg of gel slice into a 1.5 mL microcentrifuge tube.
- Add 500 μL of Buffer B and mix by vortex.
- Incubate at 60°C for 10 minutes (until gel is completely dissolved).
- During incubation, vortex every 2–3 minutes.
- Allow the mixture to cool to room temperature.
🔹 Step 2 – DNA Binding
- Insert a PG Column into a Collection Tube.
- Apply the supernatant (from Step 1) to the PG Column by pipetting.
- Centrifuge at 14,000 × g for 30 seconds.
- Discard the flow-through and return the PG Column to the collection tube.
🔁 If volume >800 μL, load and spin again.
🔹 Step 3 – Washing
- Add 400 μL of Buffer W1 to the PG Column.
- Centrifuge at 14,000 × g for 30 seconds.
- Discard flow-through and reinsert column.
- Add 600 μL of Buffer W2 (ethanol added).
- Centrifuge at 14,000 × g for 30 seconds.
- Discard flow-through and reinsert column.
- Centrifuge again at 14,000 × g for 2 minutes to remove residual wash buffer.
🔹 Step 4 – DNA Elution
- Transfer the PG Column to a clean 1.5 mL microcentrifuge tube.
- Add 50–200 μL of Buffer E or nuclease-free water (pH 7.0–8.5) to the center of the column membrane.
- Let stand for 2 minutes, then centrifuge at 14,000 × g for 2 minutes.
⚠️ If any salt precipitate is visible in buffers, warm to 37°C to redissolve before use.

Applications:
- DNA sequencing
- Restriction enzyme digestion
- Ligation and transformation
- In vitro transcription
- PCR and re-amplification
- DNA labeling
- Microinjection