Introduction
Traditional immunoassays, such as enzyme-linked immunosorbent assay (ELISA) and Western blot, provide bulk population-level measurements of antibody-antigen interactions. However, they fail to capture molecular heterogeneity, binding kinetics at the single-molecule level, and real-time interaction dynamics. Advances in biophysics and nanotechnology have led to single-molecule characterization methods that provide unprecedented insights into antibody affinity, specificity, and structural dynamics. This blog explores next-generation single-molecule techniques, their applications in antibody characterization, and their potential to redefine immunological research.
Limitations of Conventional Immunoassays
Conventional antibody characterization techniques rely on bulk averaging:
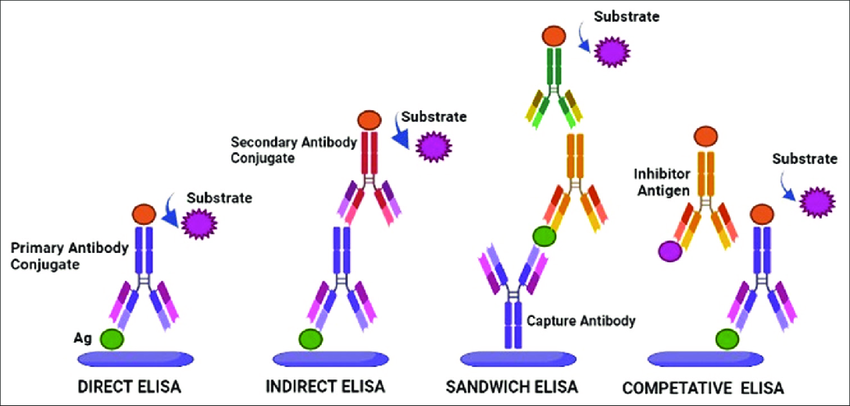
- ELISA measures antigen-antibody binding via enzymatic amplification but lacks real-time kinetic resolution.
- Western blot provides qualitative detection but lacks quantitative precision and real-time interaction monitoring.
- Surface plasmon resonance (SPR) and biolayer interferometry (BLI) offer real-time binding kinetics but suffer from ensemble averaging.
These methods cannot distinguish heterogeneous binding populations, detect rare binding events, or capture dynamic conformational changes.
Single-Molecule Techniques for Antibody Characterization
1. Single-Molecule Fluorescence Microscopy
Fluorescence Resonance Energy Transfer (FRET)
- Principle: Monitors distance-dependent energy transfer between donor and acceptor fluorophores conjugated to antibody-antigen pairs.
- Application: Resolves conformational changes in antibodies upon antigen binding.
- Advantages: Enables real-time tracking of binding-induced structural rearrangements.
- Limitations: Requires precise fluorophore labeling and high signal-to-noise ratios.
Total Internal Reflection Fluorescence (TIRF) Microscopy
- Principle: Selectively excites fluorophores near a surface (~100 nm penetration), reducing background noise.
- Application: Single-molecule binding assays for determining affinity and on/off rates.
- Advantages: High spatial resolution and surface-specific interaction analysis.
- Limitations: Surface immobilization may affect antibody binding dynamics.
Stochastic Optical Reconstruction Microscopy (STORM)
- Principle: Utilizes photo-switchable fluorophores for nanometer-scale super-resolution imaging.
- Application: Mapping antigen distribution on cell membranes and characterizing antibody localization.
- Advantages: Breaks the diffraction limit for high-resolution epitope mapping.
- Limitations: Requires specialized fluorophores and complex image processing.
2. Optical Tweezers
- Principle: Uses tightly focused laser beams to apply piconewton-scale forces on antibody-antigen complexes.
- Application: Measures unbinding forces and mechanical stability of antibody interactions.
- Advantages: Quantifies molecular forces governing antibody-antigen recognition.
- Limitations: Requires specialized optical trapping instrumentation and high laser stability.
3. Atomic Force Microscopy (AFM)
- Principle: Uses a nanoscale cantilever to scan surface-bound antibodies and measure binding forces.
- Application: Single-molecule force spectroscopy (SMFS) quantifies unbinding kinetics and affinity heterogeneity.
- Advantages: High-resolution mapping of antibody binding sites on antigens.
- Limitations: Requires immobilization, which may affect binding conformation.
4. Nanopore Sensing
- Principle: Detects single antibody-antigen interactions by monitoring ion current perturbations through nanopores.
- Application: Identifies antibody binding events, measures binding kinetics, and detects post-translational modifications.
- Advantages: Label-free detection with high temporal resolution.
- Limitations: Sensitivity depends on nanopore diameter and surface chemistry.
5. Single-Molecule Electrochemical Detection
- Principle: Measures electron transfer events associated with antibody-antigen binding at electrochemical interfaces.
- Application: Quantifies redox-active antibodies and their interactions.
- Advantages: High sensitivity and low sample volume requirements.
- Limitations: Requires redox-labeled antibodies for optimal signal generation.
Applications and Impact
Therapeutic Antibody Development
- Single-molecule characterization improves lead antibody selection by distinguishing high-affinity clones from weak binders.
- Enables detection of rare, ultra-high-affinity antibodies that bulk assays might overlook.
- Provides mechanistic insights into immune checkpoint inhibitors and therapeutic monoclonal antibodies.
Diagnostics and Biosensing
- Nanopore and electrochemical techniques enable rapid, label-free antibody detection for point-of-care diagnostics.
- Single-molecule fluorescence assays allow real-time tracking of disease biomarkers.
Structural Immunology
- Super-resolution imaging and AFM provide nanometer-scale epitope mapping for vaccine design.
- Optical tweezers reveal force-dependent immune recognition mechanisms.
Single-molecule techniques are revolutionizing antibody characterization by providing real-time, high-resolution, and quantitative measurements of binding kinetics, affinity, and conformational changes. These methods overcome the limitations of bulk assays, enabling novel insights into antibody function and therapeutic potential. The continued integration of biophysics, nanotechnology, and computational modeling will further enhance the precision of antibody engineering and immunodiagnostics.